Despite the increase, health experts remain hopeful a new wave can be avoided
CLARK COUNTY — For the first time in eight weeks, Clark County’s rate of new COVID-19 cases has ticked up slightly, moving from 88.8 per 100,000 people to 90.5 over the past week.

So far this week the number of daily new cases has continued that upward trend, including 44 new positive tests since Tuesday.
Is there reason for concern?
Yes and no, says Dr. Alan Melnick, the county’s public health officer and director of public health.
“We’re much better off than we were six weeks ago,” Melnick said during a Board of Public Health meeting on Wednesday morning, calling the current situation a plateau.
As the state moves into Phase 3 of the governor’s Healthy Washington roadmap to recovery plan, allowing higher capacity for restaurants, gyms, movie theaters and sports venues, it’s reasonable to expect that case rates might level off or even tick back up.
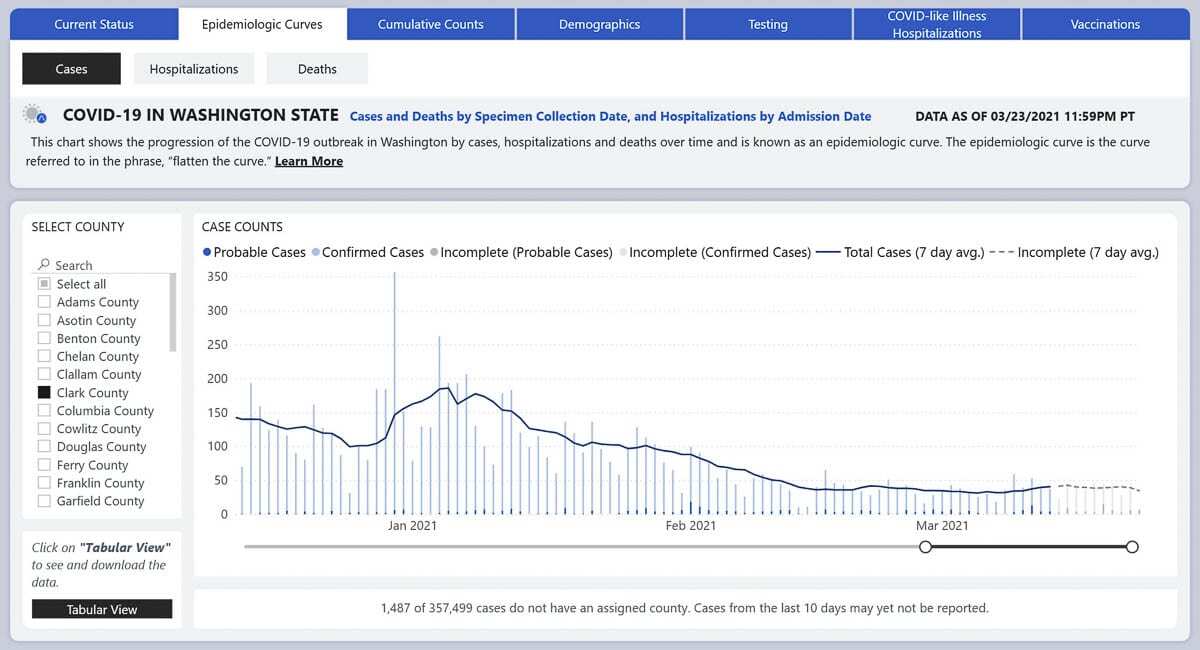
To remain in Phase 3, a county must meet two metrics: a case rate below 200 per 100,000 residents over a two week period, and five or fewer hospitalizations per day over a one week period..
“Right now, if things remain where they are, it looks pretty good for us remaining in phase three,” Melnick told the board.
The evaluation of those metrics will now happen every three weeks, with the first on April 12. Any changes to the phase a county is in would take effect the following Friday.
Melnick said vaccination efforts in Clark County have also gained ground lost earlier in the year when the state was sending far fewer doses per person here compared to other parts of the state.
“Our waiting list was over 30,000 people,” Melnick said. “We went through that waiting list pretty rapidly and basically there is no waiting list (now).”
A community vaccination site at Tower Mall in Vancouver, which is run in partnership with the city and Safeway, has vaccinated 9,000 people in 12 days.
This week that site will transition to mostly 2nd dose appointments, but will still have room for around 400 first dose appointments per day.
Area providers and pharmacies have also seen their supplies increase, and most people who are eligible for a vaccination are able to schedule appointments with relative ease.
To date, around 10 percent of the county’s overall population has been fully vaccinated, and 20 percent have had at least a first dose.
A race against time
What concerns Dr. Melnick and other medical experts is that the race to vaccinate people comes as variants of the original SARS-CoV-2 virus are becoming increasingly prevalent.
Those variants include one from the United Kingdom known as B.1.1.7, one from South Africa dubbed B.1.351, and a Brazilian variant called P.1 that has 17 unique mutations which may allow it to get around natural or vaccine-induced immunity to previous strains.
“Given the fact that we’ve plateaued, and given that we have the presence of variants here, it’s a message that we really need to not only vaccinate people as quickly as possible,” says Melnick, “but keep doing what we ought to be doing around wearing face masks, physical distancing, and avoiding gatherings.”
“I feel like at this point, we’re fighting a race,” says Dr. Amy Markezich with Overlake Medical Center in Bellevue, which has seen an increase in COVID-positive patients recently. “We’re fighting a race on getting as many people vaccinated as possible before we start to see another surge.”
Recent research has shown the UK variant was connected with a 60 percent increase in hospitalizations, raising concerns that it may not only be more contagious, but more deadly. The good news is that studies have found existing vaccines are largely effective against B.1.1.7.
Experts stress that ramping up the speed of vaccinations, which have thus far been shown to prevent infections and transmission of the virus and most of its variants, is key to slowing down the pace of mutations.
“My worry is that we might get a surge if we get too lax, or if the vaccination rates are too slow for what’s going on,” says Dr. Markezich.
Vaccine safety questioned
During Wednesday’s meeting, Clark County Council Chair Eileen Quiring O’Brien pressed Melnick on the safety of the vaccines, which have been linked to some allergic reactions and possible deaths.
“Do you have availability of reporting adverse reactions to the vaccine?” Quiring O’Brien pressed. “Because … I see headlines, and the kind of news that I get, which is not alternative, which is not social media crap. It is actual news reports of people who are having adverse effects, in fact, dying, because they’ve had the vaccine.”
Melnick responded that he would be happy to look at those reports, but that reports of even severe allergic reactions to the vaccine are exceptionally rare.
“We’ve given out a large number of vaccines, and I am completely unaware of any death in either Clark County or the state of Washington that had anything to do with the vaccine,” said Melnick.
Quiring O’Brien referred to the Vaccine Adverse Events Reporting System (VAERS), which is administered by the department of Health and Human Services in cooperation with the Centers for Disease Control. The site is open source, and anyone can report adverse reactions to vaccinations of any sort.
Through March 22, VAERS had received 2,216 reports of death following a COVID-19 vaccination, out of 126 million doses administered.
The CDC said this week that it had reviewed those reports, and concluded that “a review of available clinical information including death certificates, autopsy, and medical records revealed no evidence that vaccination contributed to patient deaths.”
According to data tracked by the CDC and FDA, a case of anaphylaxis occurred in between 2 and 5 people per million vaccinations administered. That concern, however, is why people receiving the vaccines are generally monitored for up to 30 minutes before being released. In most cases, a shot of epinephrine or other allergy medication alleviated the symptoms quickly.
Melnick noted that the AstraZeneca vaccine candidate, which is currently not approved for use in the United States, was briefly halted in some European countries due to cases of blood clotting in some recipients. Further research showed the incidents of blood clotting were similar in those receiving the vaccine and a placebo control group, and those trials resumed.
The AstraZeneca vaccine is on hold in the United States after the drug maker released data on its efficacy that a review committee alleged was based on early and incomplete data. The company has pledged to release updated information they believe will show the vaccine is up to 72 percent effective against the virus.
Vaccine eligibility should be expanded
As vaccine supplies increase, including the new on-dose Johnson & Johnson vaccination that is beginning to roll out across the country, Melnick said he is hopeful that vaccine eligibility will soon be opened up to anyone over the age of 16.
Currently, the state is planning to expand eligibility to around two million more people, or just over half of the state’s population, starting on March 30, with the goal of opening full eligibility on May 1, based on a directive from the Biden administration.
But Melnick says the easy access to vaccine appointments right now shows many of the people in currently eligible populations are choosing to either forgo the vaccination, or wait a while longer.
“The numbers that are choosing to make appointments are lower than what we’d like,” Melnick said on Wednesday. “So why not open it up to more people?”
That’s a conversation Melnick said he anticipated would be happening with health departments statewide, especially if vaccine supplies increase further and appointments remain easy to find after March 30.
“Given the confusion, and given that families would like to get vaccinated at one time, instead of sending one family member at one time, then another one,” said Melnick, “when might we look at moving towards eligibility for all adults a bit sooner, rather than waiting until May 1.”




