Despite the ongoing frustration, there are some signs of progress
CLARK COUNTY — A new slate of appointments for COVID-19 vaccinations at the Clark County Fairgrounds should open at noon on Sunday, but thousands of people are likely to again face disappointment.
It’s not because the sites aren’t working: as of this week, nearly 20,000 people had received a first dose at one of the state’s four mass vaccination sites, according to the Washington Department of Health.
There simply isn’t enough vaccine available for everyone who wants it.
This week, the 600 facilities authorized to administer vaccinations statewide requested over 358,000 first doses of vaccine. The state received only 107,125 first doses, which was actually a slight improvement over the week before.
The state was able to also provide 58,725 second doses, which was 14,000 less than providers were hoping to receive.
In Clark County, vaccination sites, pharmacies and providers were allocated 5,450 first dose vaccines this week. That’s an increase from between 1,700 and 3,700 in previous weeks, according to Clark County Public Health.
Even so, there are an estimated 89,000 people in Clark County who qualify for a vaccine under Phase 1B, Tier 1, which includes anyone over age 65, or 50 and older living in multigenerational households.
Where the vaccine is going
This week, the state also clarified how it is allocating first doses that arrive.
For this week’s shipment, 36 percent was sent to the four mass vaccination sites. Twenty-three percent went to hospitals, and 19 percent went to pharmacies. Another 19 percent went to community health centers, federally qualified health centers, local health jurisdictions, and private practitioners. Three percent went to tribes and Urban Indian Health programs.
Allocation decisions are made during a multi-step process that runs from Saturday through Thursday, in order to meet the CDC’s Friday morning ordering deadline. Enrolled providers use the state’s Immunization Information System (WAIIS) to place their order, and DOH uses information from local health jurisdictions to help determine priority levels for allocations.
Factors used in making that decision include proportional population of those eligible in the county, data from providers, current vaccine inventory of providers, and documented throughput, as well as making sure access is as equitable as possible by making supply available to providers that work with marginalized populations.
Vaccine stats to date
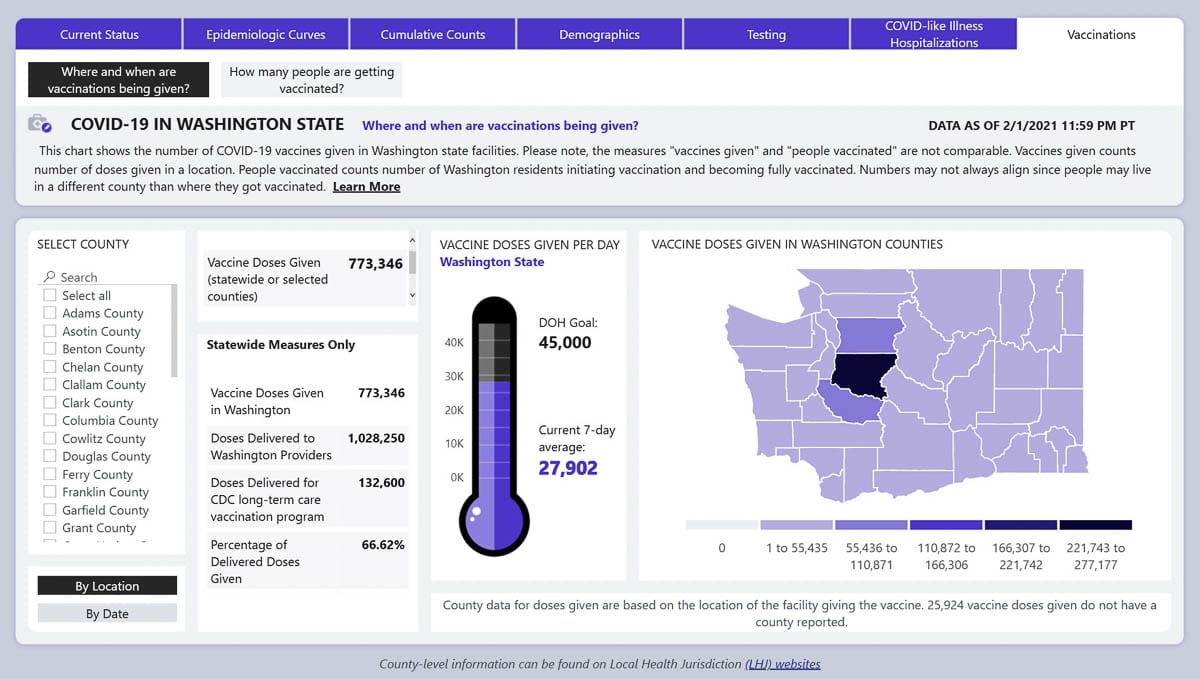
As of Feb. 1, the most recent date available, the state had administered 773,346 doses of COVID-19 vaccine, or 60 percent of the 1.16 million doses distributed to date.
Health providers have said they believe the actual percentage of doses administered is likely much higher, given delays in reporting usage through the state system.
According to the Washington State Hospital Association (WSHA), their member hospitals had reported 467,041 vaccine doses received, with all by 30,000 of those administered as of Jan. 30.
Hospitals have actually been scheduling fewer vaccine appointments as allocations shift to mass vaccination sites, and an increasing number of people cancel after finding they can obtain a dose somewhere else.
As of this week, DOH said the state was administering approximately 27,902 doses of vaccine per day. That’s actually down slightly from last week, but not an unexpected drop. Once qualifications were extended to the next phase, many hospitals quickly went through any remaining vaccine doses they had in storage.
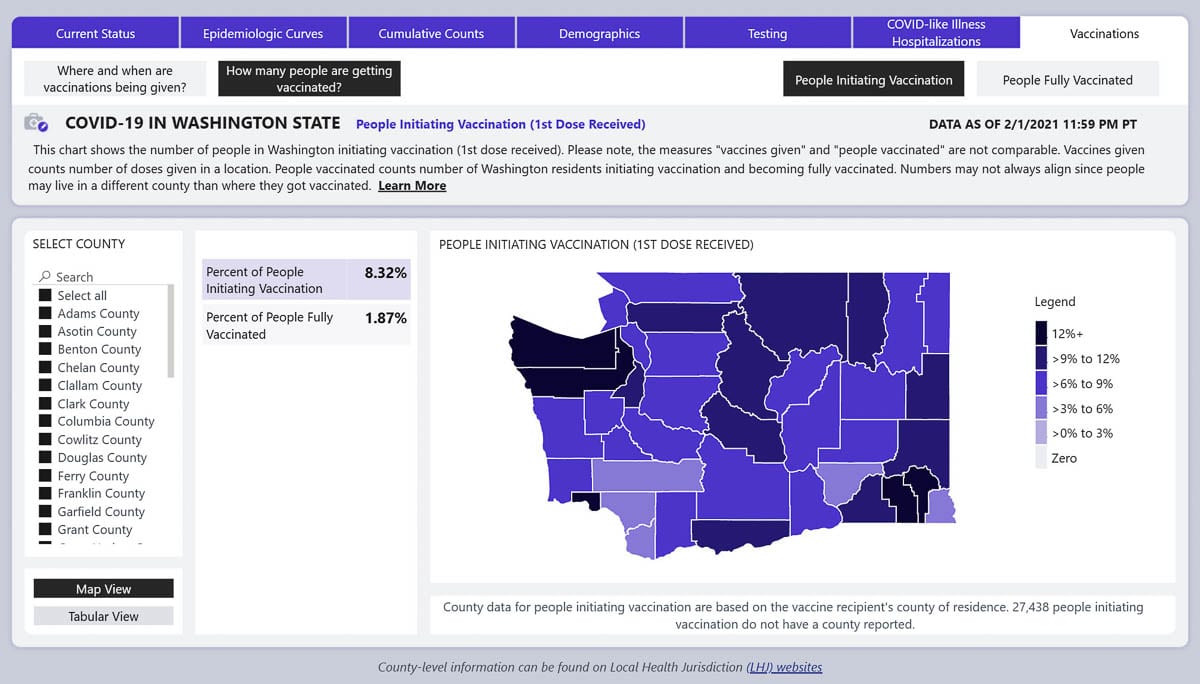
To date, 8.32 percent of the state’s population has received at least one dose of vaccine, and 1.87 percent have been fully vaccinated, according to the state’s COVID-19 Data Dashboard.
For Clark County, the number is five percent with an initial dose, and 1.23 percent fully vaccinated.
More help could be on the way
On Thursday, drug-maker Johnson & Johnson announced they were submitting a request to the Food and Drug Administration (FDA) for an Emergency Use Authorization for their single-shot COVID-19 vaccine.
If approved, the company said it could ship up to 100 million doses in the United States before the end of June.
The FDA is scheduled to meet Feb. 26 to consider Johnson & Johnson’s application, with the potential for approval in early March.
In global trials involving 44,000 volunteers, the Johnson & Johnson vaccine was shown to be 72 percent effective in the United States, and 66 percent effective overall in preventing moderate to severe COVID-19 infections.
The company claims none of the vaccine recipients died or required hospitalization within 28 days of vaccination
The vaccine is also more stable, requiring less rigid cold storage to remain viable, meaning it could be useful in remote areas where transport of current vaccines is more difficult.
Unlike the Pfizer-BioNTech and Moderna vaccines, the Johnson & Johnson candidate is a “viral vector” vaccine, meaning it uses a less harmful version of the adenoviruses, modified with COVID characteristics to help the body’s immune system recognize and destroy the actual SARS-CoV-2 virus.
The existing vaccines use messenger RNA, which contains a selected segment of the virus’ genetic code, prompting your body’s cells to replicate only the corona of the virus, which the immune system then learns to recognize and attack.




